Using Causaly to Accelerate Clinical Evidence Validation for FDA Submissions
Using Causaly to Accelerate Clinical Evidence Validation for FDA Submissions
Challenges
A Senior Medical Manager was preparing an FDA label update for a marketed drug and needed to validate outcomes from a completed clinical trial. The task required clear alignment between trial results, disease biology, and regulatory expectations.
But, the existing workflow depended on manual literature reviews and side-by-side data comparisons. Evidence was scattered across sources, requiring significant time to cross-check findings and trace outcomes back to underlying mechanisms. This slowed validation and made it difficult to confidently demonstrate biological relevance within regulatory timelines.
How Causaly Helped

Confirming Mechanism of Action and Disease Relevance
The Senior Medical Manager used Causaly to rapidly establish whether the drug’s mechanism of action had a causal relationship with the disease pathophysiology. This provided an immediate biological foundation for assessing the clinical outcomes.
Identifying Relevant Endpoints and Outcomes
Using Causaly Bio Graph, he then explored and validated the clinical endpoints relevant to the trial, visualizing how outcomes connected to disease mechanisms and supporting evidence. This made it possible to assess relevance and consistency without manually stitching together information from multiple sources.
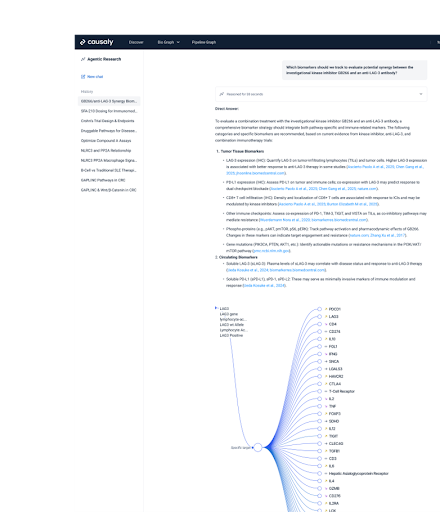

Consolidating Evidence with Agentic Research
To prepare the submission, he used Agentic Research to consolidate literature, biological evidence, and clinical insights into a single, structured, and fully cited report. This eliminated redundant review steps and ensured transparency in how conclusions were supported.
Scaling Validation Across Multiple Outcomes
Once established, the same workflow was reused across additional endpoints, allowing multiple trial results to be validated quickly and consistently without repeating manual processes.


Impact
- Causaly significantly reduced the time required to validate clinical evidence for regulatory filings, enabling faster preparation of FDA label update submissions while maintaining scientific rigor.
- Manual literature review and data comparison were replaced with an efficient, evidence-driven workflow, freeing time for higher-value regulatory and scientific review.
- Clinical outcomes could be clearly traced back to disease biology, strengthening confidence in the mechanistic rationale presented to regulators.
- Concise, citation-backed summaries improved alignment across medical, regulatory, and scientific teams, supporting more efficient collaboration.
- By applying the same approach across endpoints, the team was able to scale validation efforts and move from evidence review to submission readiness more quickly and consistently.
"I save time on my research because Causaly is easy to use. It allows me to work smarter. It would take me a longer time to get to the same amount of information. I use Causaly a lot in meetings because I can quickly get a nice summary of response for my colleagues."

More case studies

Get to know Causaly

What would you ask the team behind life sciences’ most advanced AI? Request a demo and get to know Causaly.
Request a demo

